Evolucion: Oxigeno y tamaño de los insectos por raulespert
Bienvenidos a:
domingo, abril 10, 2011
miércoles, abril 14, 2010
EPIPHYTIC GROWTH HABITS OF CHILEAN GESNERIACEAE AND THE EVOLUTION OF EPIPHYTES WITHIN THE TRIBE CORONANTHEREAE

lunes, abril 05, 2010
Behavioral development not Genetic Kin Selection!...may explain origins of sociality
It has been proposed that one of the traits that are involved in the origin of sociality is the tolerance among individuals belonging to the same species. This means that the transition from solitary to social life requires the regulation of aggressiveness and the evolution of intraspecific recognition. This is also known as kin recognition, now, when we look at the ideas to explain the origin and maintenance of this trait, there are some contrasting approaches.
The sociobiological school, propose that individuals bearing the same genes may be able to recognize themselves, this correspond to an evolutionary adaptation that allow them to collaborate and keep cohesion of related group of individual. This idea has flourished in the field of behavioral ecology and the mainstream neodarwinian culture, it has been suggested even to explain altruism in humans. However, despite the benefits suggested on the theoretical models for a genetic kin selection, these proposals are often not supported with explanatory mechanism for the occurrence of such kin recognition.
Alternatively to this approach, it have been suggested that the recognition and acceptance of individuals belonging to a defined group correspond to an emergent property due to behavioral development, where the signals present during the construction of the kin fidelity responses are molded by the available cues, these cues can originated either in the group (kin reference) or even derive from the organism itself that is developing its responses (self reference).
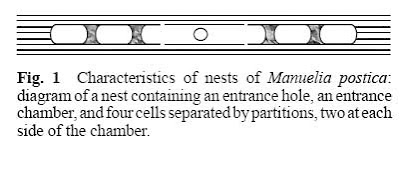
One suitable system to study the jump from solitary to social life and the origin and maintenance of intraspecific recognition is constituted by the Xylocopinae (Hymenoptera: Apidae). This group is held as the sister group for all other Apidae subfamilies, and thus it may very likely be the less derived. In addition it contains species that goes from solitary to social life in relation to nesting behavior. Among the solitary species some exhibit features more often present in social species, some of these are related to parental care, contact between related individuals and also tolerance between nestmates. Manueli apostica is one of these solitary bees, it belong to the monogenerial tribe Manuelini inside Xylocopine, a group mainly presented on continental Chile. In these bees, female construct nests inside the dead stem of Chusquea quila (Poaceae: Bambuseae), in these developing offspring are isolated by the mother in single compartments together with food supplies (Figure 1).
In this group, Dr. Luis Flores-Prado and collaborators from Facultad de Ciencias, Universidad de Chile, have found that the recognition of nestmates individuals and tolerance is either developed from cues present on food masses and nest material provided by the mother or on cues acquired by the insect themselves, also known as self referencing (Experiment 1, Figure 2). In a newly published research Dr Flores-Prado and co. performed a recognition test between two non-kin individuals (a foster and a non manipulated one, Experiment 2, comparison A) developed in the same nest, thus they will experience the same breeding environment.
Furthermore, they compared between two kin females developed in different nest, which were not used as sources of other test (Experiment 2, comparison B). So, this means same kin different environment.

These transplant design allow them to disentangle if kin recognition is achieved due to the cues from food provisioning or from cues that the bee obtain from itself. These experiments demonstrated that non-kin females developed in the same nest were more intolerant (and less tolerant) with each other than kin females developed in different nests (Figure 3). Thus, kin recognition was occurring. Individuals of M. postica are physically isolated while developing up to the adult stage prior to the destruction of cell partitions, and there is no direct contact between them and their mother after oviposition. In addition, non-volatile compounds (such as cuticular hydrocarbons) mediate nesmate recognition in this specie (at has been demonstrated by Dr. Flores-Prado). Based on these evidences the authors suggest that cues used in this “kin” recognition do not correspond to an especially unique kin cues and that kin recognition in this bee specie has occurred through self-referent phenotype matching. Just in the same way chicken embryos are able to develop its how-to-peek on a spot behavior due to the cues derived from the movements of their own organs inside the egg, one of the many amazing examples of behavioral developments discovered by Z.Y. Kuo in the beginning of the XX century.

References
Flores-Prado L Chiappa E & Niemeyer HM Nesting biology, life cycle, and interactions between females of Manuelia postica, a solitary species of the Xylocopinae (Hymenoptera: Apidae). 35:93-102.
Flores-Prado, L. Aguilera-Olivares D. & Niemeyer H.M. 2008 Nest-mate recognition in Manuelia postica (Apidae: Xylocopinae): an eusocial trait is present in a solitary bee. Proc. R. Soc. B 275, 285–291.
Flores-Prado L & Niemeyer HM 2010 Kin Recognition in the largely Solitary Bee, Manuelia postica (Apidae: Xylocopinae). Ethology 115:1–6.
Hamilton, W. D. 1964a The genetical evolution of social behavior, I. J. Theor. Biol. 7, 1–16.
Hamilton, W. D. 1964b The genetical evolution of social behavior, II. J. Theor. Biol. 7, 17–52.
Mateo, J. M. 2004: Recognition systems and biologicalorganisation: the perception component of social recognition.Ann. Zool. Fenn. 41, 729-745.
Michener CD 2000. The bees of the world. Baltimore, Maryland, The John Hopkins University Press. 913 p.
jueves, febrero 25, 2010
A pergunta que Einstein fez à Biologia
Entre as salamandras Triturus viridescens é comum a ocorrência, natural ou induzida, de poliploidia. Particularmente, o embriologista Frankhauser trabalhava na década de 40 com salamandras que podiam ser haplóides, diplóides ou pentaploides. Era uma situação muito curiosa porque existe uma relação muito linear entre o número de cromossomos e o volume celular: as células que tinham 5 vezes o número de cromossomos eram realmente muito maiores que as células haplóides. Entretanto, e aí está o curioso, Frankhauser observava que as salamandras pentaplóides ou haplóides tinha os órgãos e o corpo com o mesmo tamanho que as salamandras haplóides. Isso acontecia devido às compensações no número de células que compunham cada um dos órgãos. A figura mais conhecida deste trabalho eu mostro abaixo, que trata do diâmetro dos ductos pronéfricos. Percebam que interessante, não importa o tamanho das células, o diâmetro dos ductos pronéfricos é conservado o mesmo porque nas salamandras haplóides esses ductos são feitos de 6-8 células, mas nas salamandras pentaplóides eles são constituídos de 1-2 células. Mudam os componentes, segue conservada a forma. A condição sistêmica grita forte nesse caso!

Agora vejam que interessante: logo depois de publicar este artigo em 1945, Frankhauser recebeu uma carta do físico Albert Einstein com a seguinte colocação:
" It is really marvel, the living being. The fact alone that the thing can exist with the three or four fold crhomosome number is extremely remarkable. Most peculiar, however, for me is the fact that, in spite of the enlarged single cell, the size of the animal is not correspondingly increased. It looks as the importance of the cell as rulling element of the whole had been overestimated previously. What the real determinant of form and organization is seems quite obscure."
Parece que os problemas biológicos ficam mais claros para quem está de fora da Biologia. Que resposta temos a oferecer a Einstein, tanto tempo depois?
Abraços,
Gustavo
Referencias:
Frankhauser (1945) Maintenance of normal structure in heteroploid salamander larvae, through compensation of changes in cell size by adjustment of cell number and cell shap. J Exp. Zool.00(3) pags. 445-455
domingo, noviembre 08, 2009
Evolution and Development: A predictive science


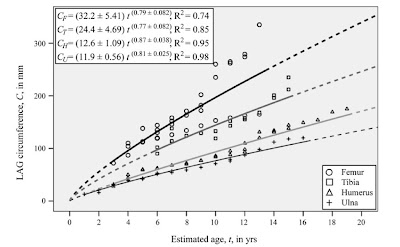
" (..) regression analyses suggest that relative to the length of the femur, the lengths of the humerus, ulna, and tibia increase in length more slowly than isometry predicts"
Traducción:
"los análisis de regresión sugieren que en relación al tamaño del fémur, las longitudes del húmero, ulna y tibia aumentan en longitud más lento de lo que predice la isometría"
miércoles, octubre 14, 2009
Symbiosis and adaptation
Associações entre plantas e fungos não são novidades; todos conhecem bem a historia das micorrizas. Mas diferente desse tipo de associação mais de fronteira entre a planta e o solo (que muitas vezes é comparado com a microbiota estabelecida em nossa pele ou intestino), há um mundo interessantíssimo de associações com fungos e bactérias que vivem entranhados no parênquima vegetal – os endófitos.
Bom, reconheço que essa associação não é uma grande novidade, já se sabe disso há algum tempo. Entretanto esta situação passou a ganhar maior atenção recentemente a partir do instante em que se observou que sempre que se fazia um PCR de extratos vegetais puros, aparecia junto a amplificação de seqüências fúngicas. Vale dizer que essa associação endofítica foi, até o presente, encontrada em TODAS, as plantas analisadas. Vou repetir: TODAS. Nesse contexto há duas situações dignas de post (e que creio ter feito a Margulis feliz, jajaja).
1) No parque nacional Yellowstone (aquele dos Geisers), existe uma flora muito particular e “perfeitamente adaptada” aos solos com altas temperaturas e alta acidez. Encontrou-se que a planta Dichanthelium lanuginosum (Fig esquerda), só é capaz de agüentar tais condições ambientais devido a uma associação que faz com o fungo endofítico Curvularia protuberata. Dissociados, nem planta nem fungo, vivem em temperaturas maiores que 38ºC! Mais interessante ainda: esta resistência parece depender de um segmento de RNA viral presente no fungo. Se o fungo não estiver infectado, não há resistência!
2) Um outro exemplo de adaptação à habitats mais extremos conferido por este tipo de associação também ocorre com uma planta de restinga, que cresce em dunas de praias. Encontrou-se que a resistência a salinidade de plantas Leymus mollis (Fig direita) nas regiões costaneiras se deve a uma simbiose com um fungo endófito Fusarium culmorum. Curioso que são plantas de dunas, adaptadas a serem plantas de dunas, mas, se se tira este fungo que está lá dentro junto ao sistema vascular da planta, elas não sobrevivem nas dunas.
Creio que estas associações são muito interessantes e muito sérias pois estão na gênese do estabelecimento de um modo de viver particular. Uma construção de nicho que depende do encontro de duas ontogêneses!
Por fim, mas não menos importante, cabe ressaltar que encontrei estes exemplos estudando imunologia, porque esta também é uma situação interessante desde o ponto de vista do adoecer. Digo isso porque se saco uma espécie de fungo que faz associação mutualista com um cultivar de tomate e o coloco (este mesmo fungo) em outro varietal de tomate (mesmo background genético), então surge uma associação parasitóide e uma planta doente. Isso nos mostra claramente que o adoecer não é causado por uma bactéria agressora e invasiva, mas sim um resultado que emana de um estabelecimento ou não de uma relação particular entre os dois organismos. Isso é muito diferente da idéia de microorganismos como agencias causadoras do adoecer – uma antiga idéia de Pasteur que ainda é muito aceita na Imunologia atual. Por esses e outros motivos queremos tanto que Biologia e Imunologia se reencontrem.
Até Dezembro,
Gustavo
viernes, octubre 09, 2009
Limusaurus and bird digit identity
Vargas AO, Wagner GP and Gauthier, JA. 2009. Limusaurus and bird digit identity. Available from Nature Precedings
Limusaurus is a remarkable herbivorous ceratosaur unique among theropods in having digits II, III and IV, with only a small metacarpal vestige of digit I1. This raises interesting questions regarding the controversial identity of avian wing digits. The early tetanuran ancestors of birds had tridactyl hands with digital morphologies corresponding to digits I, II & III of other dinosaurs2. In bird embryos, however, the pattern of cartilage formation indicates that their digits develop from positions that become digits II, III, & IV in other amniotes3. Limusaurus has been argued to provide evidence that the digits of tetanurans, currently considered to be I, II and III, may actually be digits II, III, & IV, thus explaining the embryological position of bird wing digits1. However, morphology and gene expression of the anterior bird wing digit specifically resemble digit I, not II, of other amniotes4,5. We argue that digit I loss in Limusaurus is derived and thus irrelevant to understanding the development of the bird wing.
If the extremely reduced hand morphology of Limusaurus was once present in the ancestors of birds (Figure 1A), several traits of digits I, II & III must have been lost (Figure 1A, step 1) and then re-evolved on digits II, III & IV (Figure 1A, step 2)1. The alternative is for the extremely reduced morphology of Limusaurus to have evolved in Ceratosauria, while bird ancestors retained digits I, II & III (Figure 1B). Quantitative analysis only favors the II,III,IV identification of tetanuran digits when bird digits are coded as II,III,IV, a category assumption based on embryological position alone1. This is not a truly integrative comparison, since it excludes phalangeal and metacarpal similarities that bird digits share with digits I, II and III of other theropods. When this assumption is removed, the I,II,III identification of tetanuran digits is most parsimonious1.
Rather than assume the priority of either morphological or embryological data, we propose that a homeotic frameshift occurred in the bird line, such that digits I, II, & III develop from embryological condensations 2, 3 & 46. That hypothesis has been supported by the observed absence of expression of most HoxD genes (HoxD-10, HoxD-11 and HoxD-12) only in the anterior digit of the embryonic wing, a feature diagnostic of digit I of mouse4;HoxD-11 expression in alligator is also absent only in digit I5. Experiments applying Cyclopamine (a down-regulator of Shh signaling) to the early wing bud show a frameshift of both digit morphology and HoxD-12 expression with regard to the pattern of cartilage formation, viz., anterior and middle digits now develop from positions 3 and 4, and the posterior digit normally developing from position 4 is lost7. In our scenario (Figure 1B), a similar frameshift occurred in the raptorial forelimbs of bird ancestors (Figure 1B, step 2), probably upon loss of digit IV in early Tetanurae6,7. The frameshift would be unrelated to digit I loss in the extremely reduced forelimbs of Limusaurus (Figure 1B, step 1). A few metacarpal traits of tetanurans resemble those of digits II,III & IV of other theropods1. The frameshift could have affected all but these few de-coupled traits, which may provide a morphological signature of the occurrence of the frameshift towards the origin of Tetanurae.
It is debatable whether digit morphologies can disappear and re-appear in a different position, and whether such a step-wise process could be considered a homeotic frameshift (as suggested by Xu et al.). While Limusaurus expands our knowledge of digit reduction in theropods, it does not support a strong inference that any loss and re-gain of digital morphologies has actually occurred in the lineage leading to birds.
Alexander O. Vargas1, Günter P. Wagner2 and Jacques A. Gauthier3.
1Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Santiago, Chile. e-mail: thearchosaur@gmail.com
2Department of Ecology and Evolutionary Biology, Yale University, New Haven, Connecticut, United States of America
3 Department of Geology and Geophysics, Yale Peabody Museum of Natural History, New Haven, Connecticut, United States of America
Figure 1. Alternative interpretations (A and B) of Limusaurus and the evolution of bird digit morphology. A) Limusaurus represents the morphology of bird ancestors; this implies extreme digit reduction occurred (Step 1: loss of digit I, loss and shortening of phalanges), but thereafter normal I, II, III morphologies re-appeared on digits II, III and IV (Step 2). B) Limusaurus does not represent the morphology of bird ancestors. Extreme digit reduction occurred only in Ceratosauria (Step 1); we propose that a homeotic frameshift accompanied the loss of digit IV (orange) in Tetanurae (Step 2), such that morphology and gene expression of digits I, II and III occur at embryological positions 2, 3 and 46. Colours indicate digit identity according to number of phalanges, morphology and gene expression. The number of phalanges developing at each inferred embryological position is indicated under each hand (x means complete loss of the adult digit). Image modified from Xu et al.1
- Xu, X., et al. A Jurassic ceratosaur from China helps clarify avian digit homologies. Nature 459: 940-944 (2009)
- Gauthier, J. A. Saurischian monophyly and the origin of birds. Mem. Calif. Acad. Sci. 8: 1–55 (1986)
- Müller, G.B., and Alberch, P. Ontogeny of the limb skeleton in Alligator mississippiensis: Developmental invariance and change in the evolution of archosaur limbs. J. Morphol. 203: 151–164 (1990)
- Vargas AO, Fallon JF. Birds have dinosaur wings: The molecular evidence. J Exp Zool (Mol Dev Evol) 304B: 86–90. (2005).
- Vargas, A.O., Kohlsdorf, T., Fallon, J. F., Brooks, J. V. & Wagner, G. P. The evolution of HoxD-11 expression in the bird wing: insights fromAlligator mississippiensis. PLoS ONE 3, e3325 (2008).
- Wagner, G. P. & Gauthier, J. A. 1,2,3 = 2,3,4: a solution to the problem of the homology of the digits in the avian hand. Proc. Natl. Acad. Sci. USA 96, 5111–5116 (1999).
- Vargas, A.O. & Wagner GP. Frame-shifts of digit identity in bird evolution and Cyclopamine-treated wings. Evolution & Development 11: 163-169 (2009)


